Activated carbon—also known as activated charcoal—is a remarkable substance known for its high surface area and incredible adsorption capabilities. It’s used worldwide in water purification, air filtration, medicine, and even food processing. But how exactly is this powerful material made?
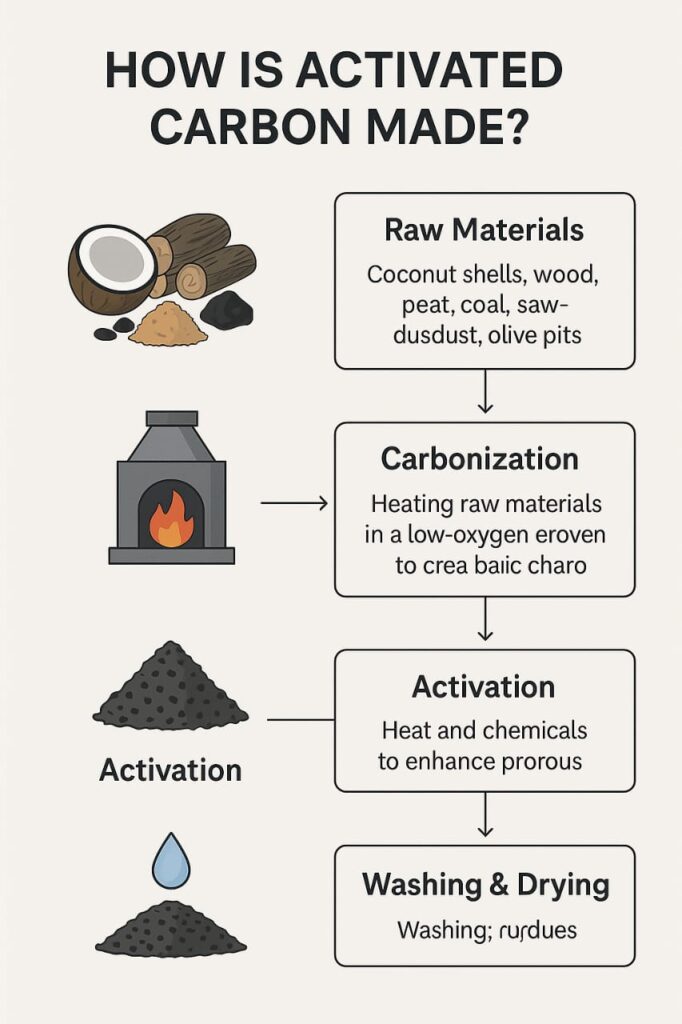
Let’s take a closer look at the step-by-step process of manufacturing activated carbon.
What is Activated Carbon?
Activated carbon is a form of carbon processed to have tiny, low-volume pores that increase the surface area available for adsorption or chemical reactions. Just one gram of activated carbon can have a surface area exceeding 3,000 m²!
Raw Materials Used
The journey of activated carbon begins with a carbon-rich organic material. Common sources include:
Coconut shells
Hardwood
Peat
Coal
Sawdust
Olive pits
Coconut shells are often preferred due to their high density and low ash content, especially for water and air purification uses.
Step-by-Step Production Process
- Carbonization
The raw material is heated in an environment with little to no oxygen. This removes volatile compounds and leaves behind char—pure carbon in a porous form. This is typically done in a kiln or furnace at temperatures between 400°C and 700°C.
Goal: Turn organic matter into basic charcoal without burning it to ash.
- Activation
The char produced during carbonization is then “activated” to enhance its porous structure and surface area. This can be done in two main ways:
a. Physical Activation (Steam Activation):
Char is heated to 800–1100°C in the presence of steam or carbon dioxide.
This process opens up tiny pores in the carbon structure.
b. Chemical Activation:
The raw material is impregnated with chemicals like phosphoric acid (H₃PO₄), potassium hydroxide (KOH), or zinc chloride (ZnCl₂).
It is then heated to around 450–900°C.
The chemical agents help dehydrate the material and open the internal structure before being washed out.
Key Advantage: Chemical activation usually requires lower temperatures and results in a higher surface area.
- Washing and Drying
After activation, the material is thoroughly washed—especially if chemicals have been used—to ensure residue removal. Distilled water or acidic solutions are used to neutralize the carbon.
Then, it is dried to remove all moisture before packaging.
- Sizing and Packaging
The activated carbon is crushed, screened, and classified into different particle sizes based on its intended final use.
Powdered Activated Carbon (PAC) – Fine powder, often used in batch treatment.
Granular Activated Carbon (GAC) – Larger particles, ideal for filtration systems.
Pelletized Carbon – Uniform cylindrical shapes, used in gas treatment and industrial applications.
Applications of Activated Carbon
Water treatment: Removes chlorine, organic compounds, and contaminants.
Air purification: Eliminates VOCs, odors, and pollutants.
Medical use: Treats poisonings, gas, and indigestion.
Food & beverage: Removes impurities and unwanted tastes.
Gold recovery: Used in mining to separate gold from ore.
Conclusion
The production of activated carbon is a fascinating blend of science and engineering. From humble coconut shells or wood, we can create a material capable of purifying water, cleaning air, and saving lives. Whether you’re in industry, research, or simply curious, understanding how activated carbon is made gives a new appreciation for this everyday wonder.
